Katherine Jarvis-Shean, UCCE Orchard Advisor Yolo, Solano, & Sacramento Counties
Allan Fulton, UCCE Irrigation and Water Resources Advisor Emeritus
Given low precipitation this past winter and low surface water allocations, many growers may be faced with the difficult prospect of using lower quality water for irrigation this season, either using more groundwater than usual or using groundwater that may have decreased in quality. What should be considered before irrigating with lower quality water?
What do we mean by “lower quality” water? And why does it matter?
Water can be low quality in a number of ways – high salinity, high sodicity, high in specific toxic ions, and/or high in bicarbonates.
Saline irrigation water can interfere with water uptake in the roots. Water moves into roots through osmosis. A high concentration of ions (dissolved salts) in the roots draws water into the roots, but if ion concentrations are also high in the soil-water solution, this root draw won’t be as powerful. The end result is decreased growth due to reduced water uptake by the tree. Electrical conductivity of the water (ECw) is a measure of the potential for this osmotic effect.
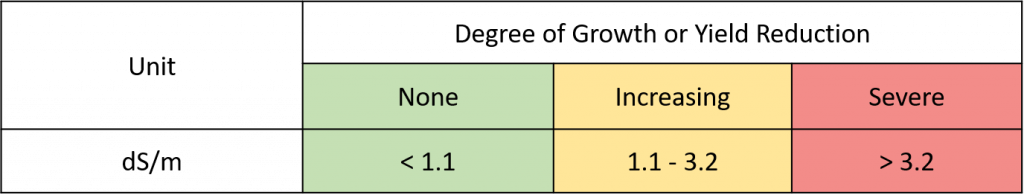
Figure 1. Does ECw pose a potential problem (osmotic effects)?
Sodicity is specific to the influence of sodium relative to other positive ions. High sodium relative to calcium and magnesium can lead to structural soil crusting. Water with a high sodium adsorption ratio (SAR) is dominated by sodium that elbows calcium off clay soil particles, destabilizing soil aggregates and causing problems with water infiltration. If the SAR/EC ratio is greater than 10, amendments are beneficial to avoid soil dispersion and sealing, either by directly adding calcium (e.g. gypsum) to the water or soil, or driving off bicarbonate and freeing up more calcium (i.e. acidifiers) to increase EC of the irrigation water and soil-water solution. Between 5-10 SAR/EC, amendments may be beneficial. If magnesium is high relative to calcium and sodium is low, magnesium may also act as a dispersant, destabilizing soil aggregates and causing slow water infiltration. If the Ca/Mg ratio is less than 1 (i.e. more Mg than Ca), this could be a red flag.
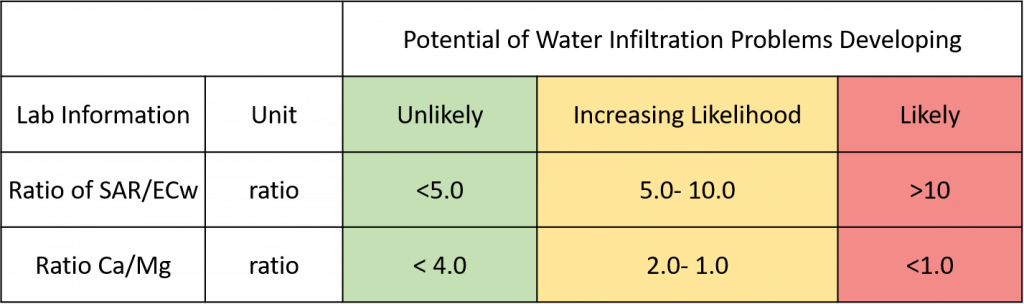
Figure 2. Could the water chemistry reduce soil tilth, porosity, and cause slower water infiltration rates?
Toxicity from specific ions occurs when these ions move into the plant with water and accumulate in plant tissue to a level that kills the tissue. Depending on your region, sodium (Na), boron (B), and chloride (Cl) could be at toxic levels in some water sources.
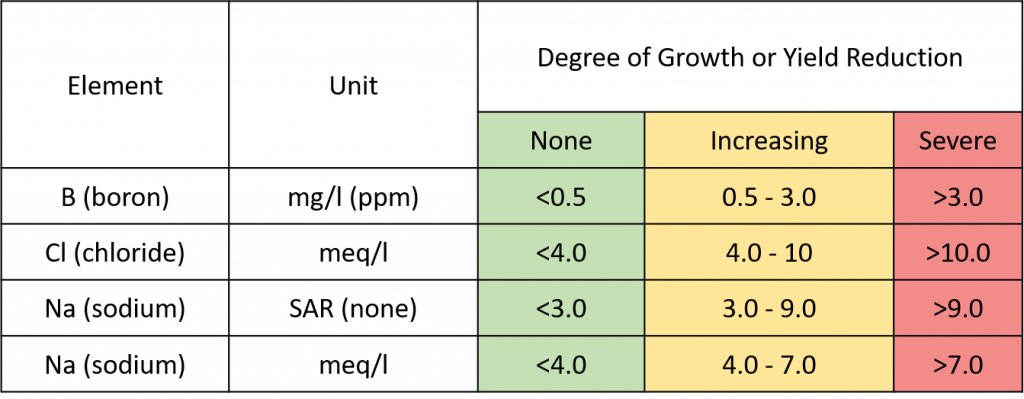
Figure 3. Could specific elements (B, Cl, and Na) accumulate in the tree or soil to potentially toxic levels?
Irrigation water high in bicarbonates (HCO3), greater than 2 meq/l, precipitates Ca with HCO3, forming lime which can clog drip emitters, microsprinklers and filters, leading to issues with irrigation distribution uniformity, with some trees getting over- or under-irrigated, or a mix of both across an orchard. High bicarbonates may also influence balances between Ca, Na, Mg, and K but there is much to still be learned on this subject.
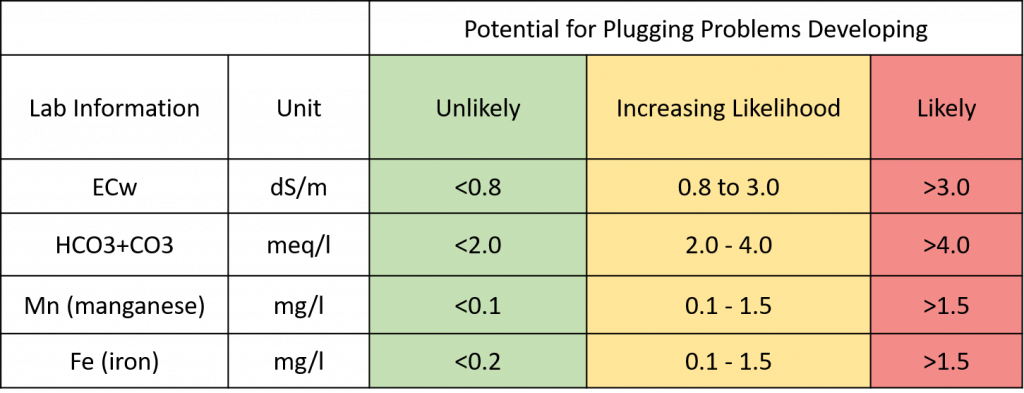
Figure 4. Could the water chemistry be prone to plugging emitters or filters and reduce distribution uniformity?
Guidance here can help to understand if the water sources you are considering have quality problems that may need to be dealt with.
What to watch for this year?
Before you make any decisions, get a sense of where you’re starting from. Without a large amount of heavy, drenching rain this year, there was not much leaching of specific ions from the root zone. With each irrigation, sodium, boron and chloride can build up over the growing season. If you are concerned about toxic ions and considering using lower quality water this year, it would be prudent to test your soils around the root zone to see the baseline specific ion levels with which you are coming into the season. Make sure you test different depths in the root zone, ideally to at least three feet. It’s important to understand the distribution of salts in the root zone. They may have moved down the soil profile but not all the way out of the root zone. Also consider reviewing leaf (Cl and Na) and hull (B) analysis results from last year.
As you consider the short- and long-term potential impacts of using lower quality water this year, think through what tools are at your disposal to mitigate those potential impacts. If you are worried about increased SAR or low Ca/Mg ratio leading to soil crusting, what are your options for adding calcium to the water or soil? What options do you have to add acidic amendments to lower bicarbonates or lower soil pH to liberate calcium from lime when it’s present in the alkaline soils? Running these amendments in your irrigation water is generally the most efficient, direct approach, especially with drip systems. Even though these amendments may be more expensive, they will be going directly to the point of the problem, rather than cheaper broadcasted products that may improve infiltration in the middles, far from where your irrigation water touches. See this article for more on product purity and calculating amendment rates.
If you are concerned about lower quality water leading to salt build up in the root zone and reducing yield, how easy or difficult will it be to leach those salts this fall/winter? Do you have a clay layer or other soil profile issues that would make leaching difficult? Whereas soil crusting can happen with just a few irrigations and be easily perceived with the eye, root zone salinity build up may happen slowly over time. This is why having a beginning of season soil baseline that you can compare against at the end of the season is valuable, to know if leaching intervention after the end of the season is warranted.
When you have two sources of irrigation water, one better quality than the other, you have the options to irrigate with them separately or blend them into one source. If you choose to use them separately, keep in mind that early stages of growth are more likely vulnerable to stress related to water quality, particularly the osmotic root zone salinity stress that can result in plant tissue being water stressed even though there is ample water in the root zone. If you are more interested in blending the two sources of water into one supply, you can find some help with blending water calculations here.
As we go through the season, if pumping depths change, water quality from the same well can change. Rather than paying for multiple water tests throughout the season to know if you have a problem, you can keep an eye on the EC levels with a portable EC meter (~$50). Use this to monitor the total salinity of the well water every 4 to 8 weeks. If total salinity (ECw) increases by 20 percent or more, submit a new sample for lab analysis to include SAR and specific ions.
If your water samples indicate specific ion levels that may eventually exceed healthy levels in your trees, be sure to take leaf samples to monitor impact mid-season for sodium and chloride and hull samples for boron. Compare your values with previous years and hold onto them in case the next couple of years are lean water years, too. It is important to compare your tissue samples against critical values (Follow this link for more information on critical values). But it is also valuable to watch how management changes like changing water sources influence these levels over time, and if additional adjustments are needed. A similar approach is prudent for soil sampling, especially if you continue to rely on lower quality water for many years. How frequently you should sample soil depends on your starting baseline and how marginal your water quality is, but generally sampling every three years should be adequate.
Summary
We’re going into the season with the water we have, not necessarily the water we want. Water quality may not be ideal, but with careful planning and consideration many of the risks associated with low quality water can be managed in the short-term: one year to a few years.


Leave a Reply